Vaccination has been at the forefront of discussions across shipping as a major factor linked to the unprecedented crew change crisis, as vaccinated seafarers could have fewer travel restrictions making crew changeovers easier.
COVID-19 vaccines help our bodies develop immunity to the virus that causes COVID-19 without us having to get the illness. As of 26 May 2021, a total of 1.546.316.352 vaccine doses have been administered globally, according to WHO. (Find an interactive map here.)
It’s not vaccines that will stop the pandemic, it’s vaccination,
…WHO (2020).
How quickly seafarers are to be vaccinated mostly depends on the nationality of the seafarer. At present, vaccines are primarily available from government agencies with each country having its own vaccination plan.
Recent data showed that some of the key crew supply nations may not have completed their vaccination programs until 2023, which means that many seafarers could not be vaccinated until 2023 at the earliest as the majority of crew are below 50 years old and are less likely to be considered in the vulnerable category.
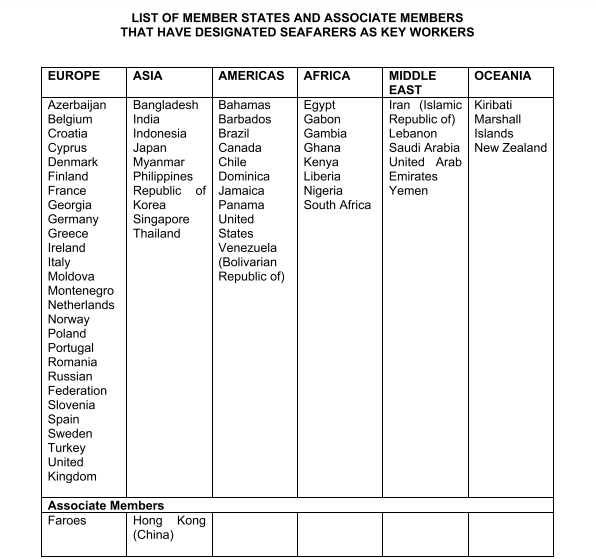
This has led several shipping organizations, unions, and associations to issue repeated calls to action to governments to recognize seafarers as key workers and thus prioritize them for COVID-19 vaccination. To date, a total of 58 out of the 174 IMO Member states have done so.
[smlsubform prepend=”GET THE SAFETY4SEA IN YOUR INBOX!” showname=false emailtxt=”” emailholder=”Enter your email address” showsubmit=true submittxt=”Submit” jsthanks=false thankyou=”Thank you for subscribing to our mailing list”]
However, the shipping challenge is rather easy to resolve as it forms part of a wider challenging situation regarding vaccine supply. Some of the world’s richest nations have pre-ordered billions of doses of vaccines – enough to protect some populations several times over, thus leaving less for others, leading to a phenomenon called “vaccine nationalism”. A similar pattern was seen during the 2009 H1N1 influenza pandemic. Vaccine nationalism is a term reflecting the tendency of countries to prioritize their own vaccine needs, impacting not only the global health recovery but the economic one, too.
“This global competition for vaccine doses could lead to prices spiking exponentially in comparison to a collaborative effort such as the COVAX Facility,” World Health Organization chief Tedros Adhanom Ghebreyesus said this year. The COVAX Facility, co-led by CEPI, Gavi and WHO, alongside key delivery partner UNICEF, is the global pooled procurement mechanism for COVID-19 vaccines through which COVAX will ensure fair and equitable access to vaccines for all 190 participating economies, using an allocation framework formulated by WHO.
Types of vaccines
Currently, there are three main types of COVID-19 vaccines authorized in the US, according to CDC:
- mRNA vaccines contain material from the virus that causes COVID-19 that gives our cells instructions for how to make a harmless protein that is unique to the virus. After our cells make copies of the protein, they destroy the genetic material from the vaccine. Our bodies recognize that the protein should not be there and build T-lymphocytes and B-lymphocytes that will remember how to fight the virus that causes COVID-19 if we are infected in the future.
- Protein subunit vaccines include harmless pieces (proteins) of the virus that causes COVID-19 instead of the entire germ. Once vaccinated, our bodies recognize that the protein should not be there and build T-lymphocytes and antibodies that will remember how to fight the virus that causes COVID-19 if we are infected in the future.
- Vector vaccines contain a modified version of a different virus than the one that causes COVID-19. Inside the shell of the modified virus, there is material from the virus that causes COVID-19. This is called a “viral vector.” Once the viral vector is inside our cells, the genetic material gives cells instructions to make a protein that is unique to the virus that causes COVID-19. Using these instructions, our cells make copies of the protein. This prompts our bodies to build T-lymphocytes and B-lymphocytes that will remember how to fight that virus if we are infected in the future.
In general, vector vaccines are easier to manage than mRNA vaccines, which need to be stored at very low temperatures.
What are the key available COVID-19 vaccines?
| Vaccine Brand | Who can get this vaccine? | How many shots? | When are you fully vaccinated? | WHO-approved | Type | ||
|
EU |
US |
Pfizer-BioNTech (US) | People 12 years and older | 2 shots – Given 21 days apart | 2 weeks after your second shot | Yes | mRNA |
| Moderna (US) | People 18 years and older | 2 shots – Given 28 days apart | 2 weeks after your second shot | Yes | mRNA | ||
| Johnson & Johnson’s Janssen (US) | People 18 years and older | 1 shot | 2 weeks after your shot | Yes | Vector | ||
| Oxford-AstraZeneca (EU) | People 18 years and older | 2 shots – Given 4-12 weeks apart | 15 days after your second dose | Yes | Vector |
However, national regulatory authorities have granted emergency use authorizations for as many as 16 COVID-19 vaccines, including Russia’s Sputnik V, Sputnik Light, EpiVacCorona, and CoviVac, China’s Sinopharm-BBIBP, CoronaVac, RBD-Dimer, Convidecia and Minhai, India’s Covaxin, as well as Kazakhstan’s QazCovid-in.
Risks of vaccine
As with any vaccine, the COVID-19 vaccines have been associated with some temporary side effects in some cases, such as injection site pain or tenderness, tiredness, headache, muscle pain, fever and chills. Most of these effects are mild and temporary, going away within 1-2 days.
There have been also more rare side effects reported after receipt of AstraZeneca and Johnson & Johnson vaccines, such as thrombosis with thrombocytopenia syndrome (TTS). Most cases occurred 4-20 days after receipt of the AZ vaccine, and in women under 60 years old. Some cases have also occurred after receipt of the J&J vaccine. Current data show the rate of TTS is about 6 cases per million people vaccinated, but 20-40 cases per million in those under 50 years of age.
Although initial media reports described older females to be at higher risk of developing this condition, at present there is no clear signal of risk factors that would predispose an individual to TTS, UN data suggest.
FIND A UN INTERIM GUIDANCE FOR THE DIAGNOSIS AND MANAGEMENT OF TTS HERE
A timeline of recent developments in shipping
March
- ICS issues a practical guide to tackle the spread of vaccine misinformation among crews.
- The heads of five UN organizations – ICAO, ILO, IMO, WHO, IOM– issue a joint call for seafarers and aircrew to be prioritized for COVID-19 vaccination.
- USCG issues FAQs on COVID-19 vaccines.
April
- ILO adopts a resolution for a global seafarers vaccination program proposed by the Cyprus Shipping Deputy Ministry, calling for a mapping exercise to identify the number of vaccines required for seafarers ashore at seafarer supplying countries.
May
- NSW Health announces it would provide COVID-19 vaccinations to a small number of foreign seafarers onboard vessels that transport gas between Australian ports.
- The Netherlands announces that 49,000 Janssen vaccines will be made available for seafarers from mid-June in numerous large ports and at Schiphol.
- The French government announces that French seafarers will receive priority access to Covid-19 jabs at national vaccination centres.
- By mid-May, 16 states in the US had begun vaccination programmes for non-native crew delivering goods in their ports.
- IMO’s Maritime Safety Committee (MSC 103) on 5-14 May adopts a resolution urging states to prioritize seafarers in national COVID-19 vaccination programmes.
- ICS releases a “Vaccination Roadmap framework”, aiming to boost the establishment of vaccination hubs dedicated to seafarers across the world.
- InterManager agrees with Johnson & Johnson for 1m one-shot Covid-19 vaccine doses for seafarers.
- The North American Maritime Ministry Association (NAMMA) compiles information on COVID-19 vaccine availability for foreign seafarers in US ports.
- On May 14th, Blue Planet Shipping announces that the entire crew of MV Afros was vaccinated onboard with the USA/EU approved single dose Johnson & Jonhson vaccine.
- In late May, Philippines Maritime Industry Authority (MARINA) releases guidance focusing on the vaccination opportunities for Filipino seafarers.